 |
|
| |
|
|
| Slide |
109 |
Interventions for Tuberculosis Control and Elimination |
 |
Next |
 |
 |
Previous |
 |
First |
 |
Last |
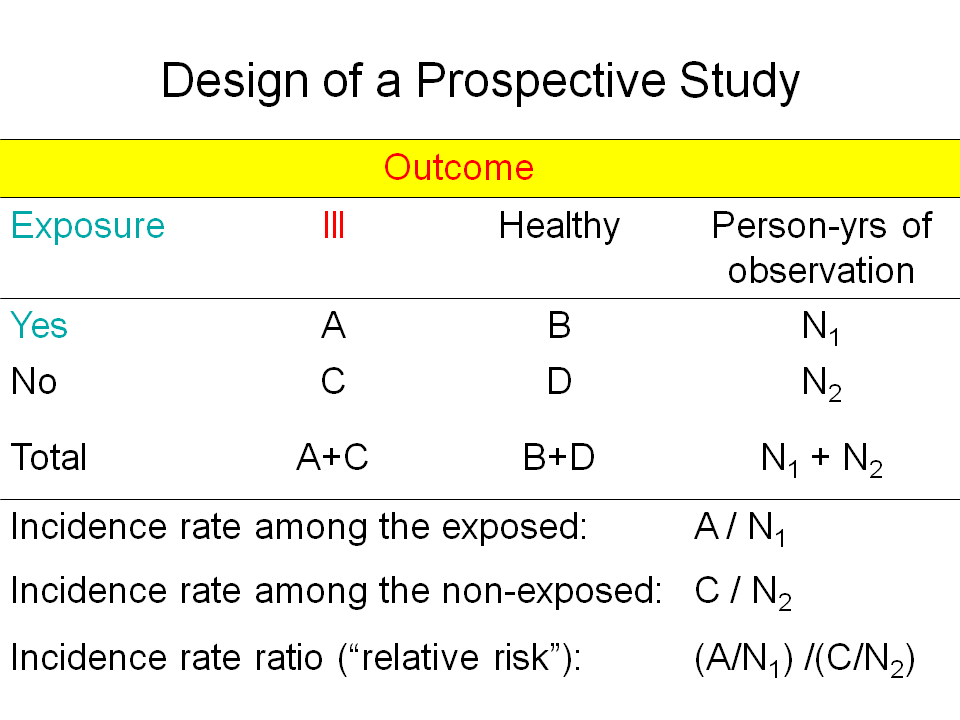
The approach to study the protective effect of BCG might be prospective or retrospective.
Prospective studies, with the randomized clinical trial as its main design start with exposure and evaluate after a defined period the outcome.
People may be randomly assigned to receive (being exposed to) BCG or a placebo. The two groups are then followed up for the occurrence of tuberculosis. The measurement of risk is the number of cases per observation time in the two groups. The relative risk that compares the two rates is the rate among the exposed divided by the rate among the unexposed. Protective efficacy is simply 1 minus the relative risk, often expressed as a percentage.
Prospective clinical trials are the gold standard for measuring vaccine efficacy but they have the disadvantage of being time-consuming and expensive to conduct. |
| |
|
Go to top
Last update:
September 29, 2010